FOUNDATIONS
The Foundation: What Are Biological Medicines?
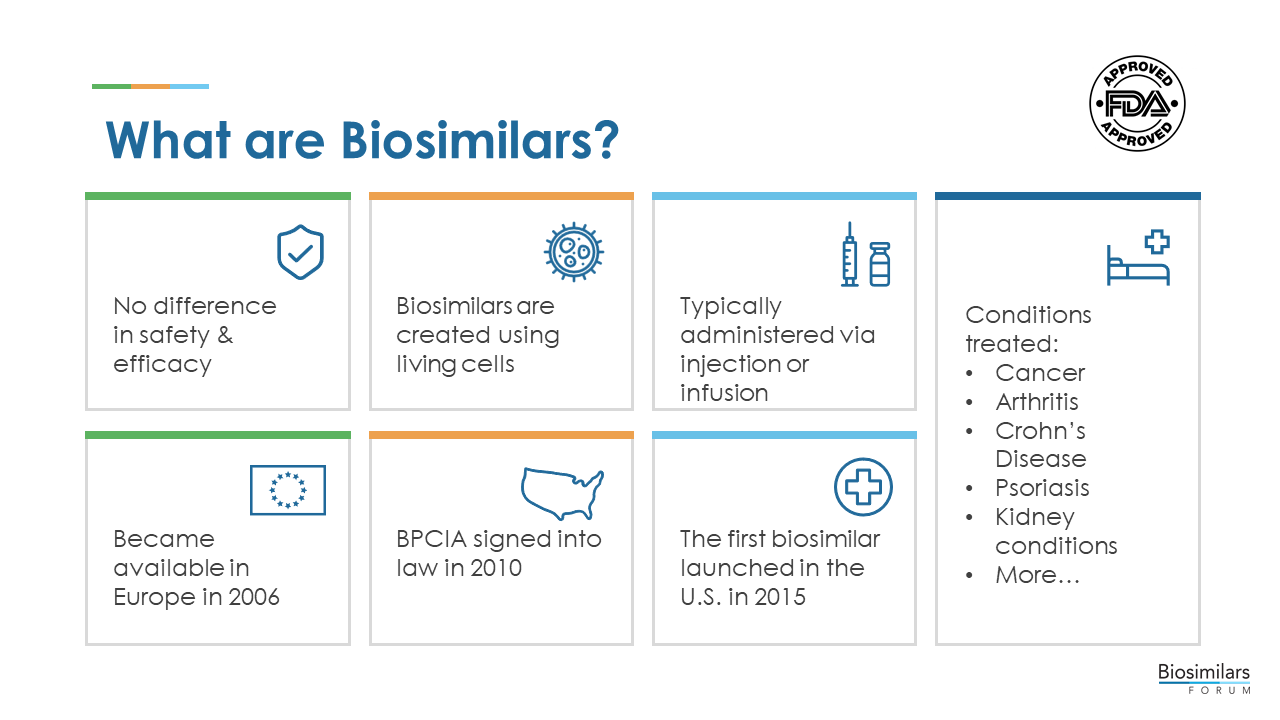
Biological medicines, also known as biological therapies or “biologics,” are medicines that are produced by living organisms, including human, animal, or microorganisms.
Biologics are more complex than traditional, chemically synthesized small molecule medicines in a number of ways, including size, three-dimensional structure, inherent variability, and manufacturing processes. While the first biologics were commonly manufactured from animal tissues, many biologics have been produced through recombinant DNA technology since the 1980s. This process uses living cells to manufacture proteins, nucleic acids (including mRNA and DNA).
All biologics vary slightly, within a batch and from batch to batch even when the same manufacturing process is used due to the inherent variability that occurs when using a natural process to produce medications. These differences are normal and expected. The process and product are closely monitored, and rigorous manufacturing controls are put in place, to ensure that all variability stays within pre-specified and pre-approved ranges to ensure that the clinical benefit and safety remain unchanged.
Biologics are more complex than traditional, chemically synthesized, small-molecule medicines.
Biologics generally treat a specific disease or disorder by supplementing or interrupting a patient’s naturally occurring biochemical pathways, processes, and signals. Some of the most difficult-to-treat diseases, such as cancer, diabetes, anemia, and autoimmune disorders (e.g., multiple sclerosis, rheumatoid arthritis, psoriasis, and inflammatory bowel disease) are often treated with biologics.
Many biologics are delivered in a health care setting in the form of an injectable or in a solution that is administered intravenously, and some biologics are self-administered by the patients or administered by a caregiver at home.